January 27, 2026 | Eric Southwell, CMO at Supreme Group
The Power of Community-First Marketing in Rare Diseases
For healthcare and pharma companies, what does it truly take to connect with people living with a rare disease in a way that’s authentic, respectful, and meaningful?
To help answer this question, I sat down with Supreme Pod co-host Paul Avery to speak with Pivot Design President and Executive Creative Director Liz Kanter as part of our latest episode. During our conversation, we unpacked the intricacies of rare disease marketing and what it takes to genuinely engage people living with a rare disease.
Why is marketing in rare diseases so challenging? The short answer is patient population size. A rare disease is defined by its low prevalence; in the United States, the disease usually affects fewer than 200,000 people, and in the European Union, no more than 5 in 10,000 individuals. These figures stand in stark contrast to common disorders like Type 2 diabetes or hypertension, which typically impact millions of people across the globe.
Such disparities in population size pose a unique challenge for healthcare marketers. For a rare disease, the condition might only involve a few hundred patients across an entire country. Given this, communications strategies built to reach broad patient populations through mass media campaigns and more generalized messaging will not work for rare disease marketing.
Instead, to resonate and build trust with rare disease communities, brands must move away from marketing strategies optimized for large audiences and adopt a different set of approaches tailored to empathy, intimacy, and connection on a one-to-one level.
In our latest episode, we unpack how to achieve this successfully. You can watch the episode in full above, or read the blog post below for a brief summary.
The Pharma Marketing “Sea of Sameness” and Why It Fails Rare Disease Communities
Blue. Spend any time reviewing pharma marketing campaigns and it is likely the main color you will see. At the same time, you might notice that most of the imagery is soft, safe, and generic, full of scenes with happy, smiling faces or people holding hands on a beach.
Why is this the case? Mainly because pharma marketing teams often default to these choices to help minimize regulatory risk. They want to make people feel safe and reinforce trust in the brand.
Safe, however, quickly becomes “same”, resulting in what Liz calls the "sea of sameness." In other words, the pharma industry drifts toward a homogeneous collection of brands and campaigns that look, sound, and feel interchangeable. In rare disease marketing, where communities are small and often feel poorly served and misunderstood, this sameness does more than cause attention blindness or trigger apathy – it can actively block a genuine connection with your audience.
This is a particular problem in rare disease marketing, where communities look for clear signs that you understand their lived experience. Patients and caregivers have spent years advocating for themselves or their loved ones, learning the science, challenging clinicians, and navigating the healthcare system, often in a world where many companies and healthcare providers don’t really understand their disease or their needs. As such, they pick up very quickly when a brand relies on generic messaging or inappropriate clichés.
Rare disease communities also tend to operate with an internal culture that is radically different from what you see in larger, more diffuse patient populations. These are communities where people often know one another by name. They might be Facebook friends or interact frequently on message boards, community subreddits and even at in-person events. As these social circles are small, messages are easily amplified, discussed, and remembered, especially if they do a poor job of reflecting the community's challenges and needs. As Liz put it during our conversation:
“It is just at a different level because you're talking about this feeling where [people] feel very alone. If you're one of 500 [people with a rare disease] in the US, you're sort of going, "Gosh, I don't want to be defined by this disease." How can we support that and make them feel like they're not?”
Acknowledging those emotions means refusing to shy away from difficult truths or uncomfortable realities. With this in mind, Liz highlighted that no emotion is off-limits at Pivot as long as “it’s real and authentic” and it has been validated by the community. Validation is a key word here, as listening to the community and reflecting their internal conversations becomes the bridge between what people live through and what shows up in a creative campaign.
Within this framing, the question shifts from “Will this make us look too negative?” to “Does this sound like what patients and caregivers care about and are actually experiencing?” Once you start from this question, the role of the marketing team changes. Rather than steering away from harder conversations, you listen for them and build around them. You can then use the complex needs of the rare disease community to design tools and campaigns that are authentic, specific, and human.
While such an approach often brings better results, it can also bring its own creative challenges. When concepts feel too safe, polished, or generic, you are forced to pause and interrogate the work. For example, internal campaign reviews at Pivot often require difficult questions and conversations: “Is this authentic? Will it empathize with the community?” If not, the idea goes back to the drawing board.
Once campaigns are grounded in that level of authenticity, your goal stops being to protect patients from difficult emotions and instead becomes supporting them through those emotions. You validate their experiences, show the full emotional landscape rather than a narrow slice of it, and let patients and caregivers genuinely see themselves in your campaigns.
When rare disease communities feel seen in that way, they are far more likely to lean in, engage, and ultimately trust your brand.
The Power of Community-First Marketing
Breaking out of the "sea of sameness" in pharma marketing and authentically supporting rare disease communities is the foundation of Pivot's creative process. As Liz explained during the episode, Pivot’s strength lies in its ability to deeply understand and empathize with rare disease communities; that’s their “get it” factor:
“It goes back to meeting patients and communities at the right altitude and the appropriateness of those solutions. It's about empathy and connecting with them at the right place, the right time, in the right way … That "get it" factor. It's not being afraid of the tagline or something more provocative that's gonna have the community remember it and go, "Wow. That pharmaceutical company gets me. They understand.”
“Getting it” in rare disease marketing means putting the community first. That requires more than a mass media, omnichannel campaign; it demands ongoing listening, co-creation, and showing up where and how the community needs support. To help us better understand, Liz described four core practices that bring community-first marketing to life:
- Get close to the community
- Leverage social listening
- Co-create with the community
- Leverage real-life success stories
Let’s look at each of these in a bit more detail and unpack all of the advice shared by Liz during the podcast episode.

1. Get Close to the Community In Person and Online
During the podcast episode, Liz suggested that pharma and healthcare companies should aim to consistently attend community events like conferences, webinars, advocacy walks, and chapter meetings. Taking this approach, your teams can directly engage with patients and caregivers, showing the community that you intend to support them continuously, not just during a product launch or peak campaign windows.
Direct community engagement in this way also provides high-touch value and can be a lot more grassroots and inexpensive than you think. For instance, Liz suggested connecting with the community at a conference by helping them “make a custom bracelet that has the name of their loved one on it or something just very tactile” to establish a direct, personal connection. This type of interaction doesn't need blockbuster budgets to truly resonate, but it does require thought, creativity, and empathy.
2. Use Social Listening to Uncover Insights
Rare disease communities maintain highly active advocacy networks online, so effective social listening can allow marketers to understand the genuine conversations that shape the daily lives of their target audiences. To get the best results, Liz suggested marketers join patient message boards and online communities "to get insights that are the real deal."
Furthermore, we recommend having conversations with a key opinion leader (KOL) or a healthcare professional (HCP) within the community. These conversations are vital for gathering information on the specific challenges faced by patients from a clinical perspective, including what it's truly like to treat a particular rare disease and what functional tools HCPs may be lacking.
3. Co-Create Tools and Resources with the Community
During the episode, Liz recommended partnering with patients, caregivers, HCPs, and child life specialists when developing new resources, emphasizing that "the best work gets done" when the community helps shape the ideas.
This co-creation process also helps teams identify when existing materials need to be retired and when new tools must be evolved to address unmet needs. Instead of creating static assets, co-creation ensures that your community support evolves alongside their lived experience.
4. Use Success Stories from Previous Campaigns to Secure Internal Buy-In
Moving to a deeply authentic, community-focused approach can bring its own risks, especially for Medical-Legal-Regulatory (MLR) review teams. So how can you get internal buy-in when trying to launch a campaign that drifts away from the safe “sea of sameness”?
When pitching bolder creative concepts internally, come armed with evidence from previous community-first campaigns. Show MLR teams how past work that pushed beyond the "safe" option actually resonated with patients and drove meaningful engagement. Real quotes, measurable outcomes, and documented community feedback from earlier initiatives create a compelling case that authentic messaging isn't just creatively satisfying; it delivers results while remaining compliant.
This track record matters because community-first marketing fundamentally changes how patients and caregivers perceive your brand. Many approach pharmaceutical companies with understandable skepticism, expecting interactions that feel transactional rather than supportive.
However, when you can demonstrate that previous community-centered campaigns built genuine trust and loyalty, you give reviewers confidence that the approach works. As Liz explained during our discussion, authentic engagement shifts perception from "Big Bad Pharma" to support that feels "really, really real" – safe and empathetic. Success stories from past campaigns become your strongest argument for continuing to put the community first.
A Pivot Design Case Study: How Therapeutic Play Became a Movement
Perhaps my favorite part of the conversation with Liz was a story that really brings the concept of community-first marketing to life.
Fifteen years ago, a client approached Pivot with a clear challenge: how could they better support children living with primary immunodeficiency?
Primary immunodeficiencies interfere with how the immune system functions, leaving patients vulnerable to frequent, severe infections. For many patients, life involves regular, time-consuming intravenous infusions. These treatments are often intimidating, exhausting, and frightening, especially for children.
Market analysis highlighted that parents and HCPs were observing a consistent pattern where children with primary immunodeficiency were scared of the needles, the infusion centers, and of being different from their peers. To avoid more fear and pain, many of the children simply stopped verbalizing their feelings altogether.
Working together, the Pivot-client team partnered with child life specialists, caregivers, and children to listen, learn, and brainstorm solutions. Through those conversations, one idea kept resurfacing: therapeutic play.
A therapeutic play kit is a non-threatening, child-friendly vehicle that helps the children process their disease state, articulate their feelings, and normalize their experience. It also empowers them to better understand their condition and gain a crucial sense of control around their treatment.
From this starting point, a blue stuffed bear named IGI emerged.
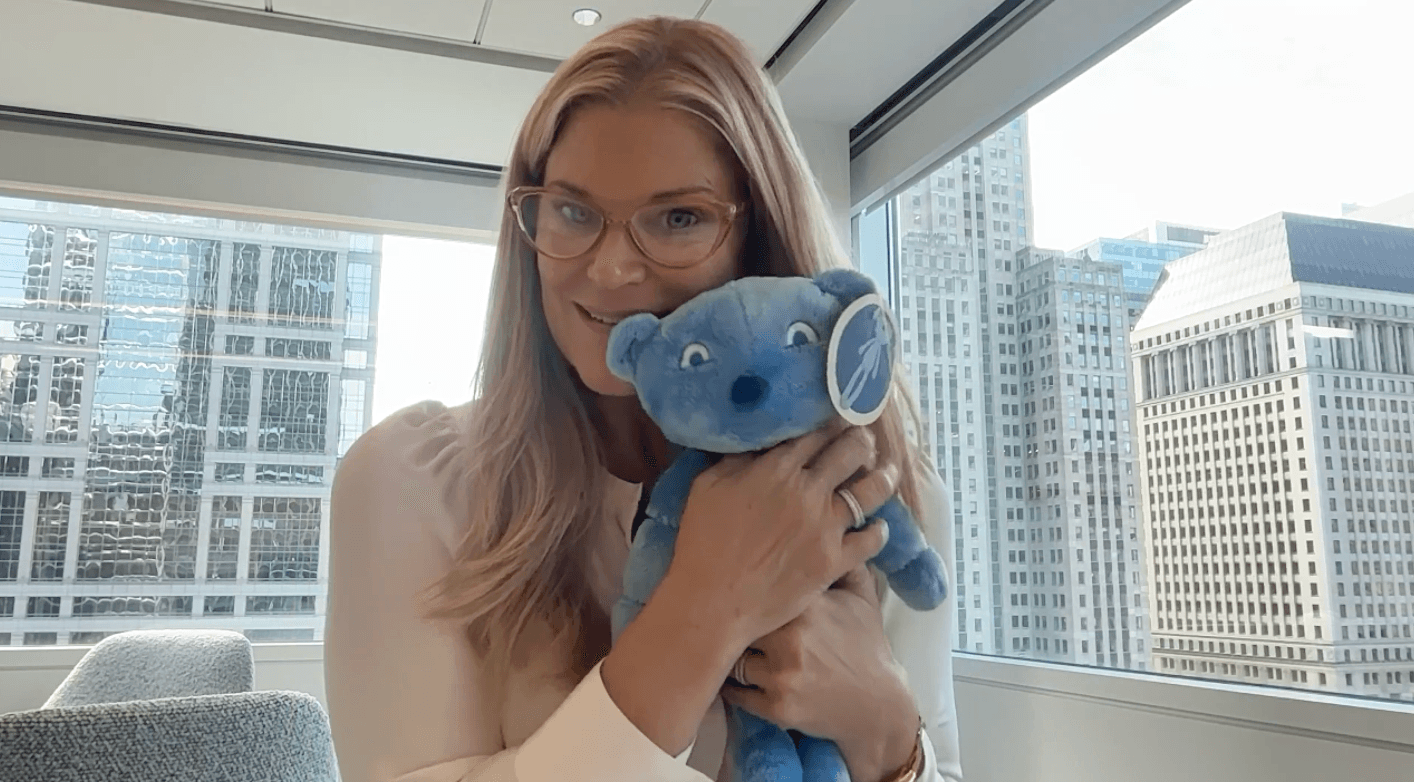
This was not a generic toy. IGI was a comprehensive tool tailored to the primary immunodeficiency experience. He came complete with a detailed medical play kit, including an infusion bag, IV tubing, bandages, and books. Describing the bear’s critical role, Liz explained, "He’s a patient’s friend. He joins patients at infusions."
IGI’s first public appearance was at a patient conference and the response was immediately positive. Excited, smiling children carried their bears through the halls and “it was the most magical, wonderful moment to see these kids' faces to get their stuffed animal and their bag of infusion supplies and see that other kids were just like them.”
IGI quickly became a shared movement. This tool, designed to make infusions less frightening for children, became a highly visible symbol of solidarity and comfort. Inspired by the original toy, an adult-sized mascot version of IGI was developed, which joined the primary immunodeficiency community on advocacy walks, attended events, and helped to rally internal teams.
An adult-sized IGI proved even more impactful than the original toy. Many patients wanted to meet IGI in person, which led to a sweepstakes to win a visit from the bear.
During the sweepstakes, Liz and her team learned of a young boy with severe primary immunodeficiency, who was living in nearly complete isolation. Arranging a visit for him required extensive documentation and MLR approvals; this would be a huge logistical lift for Pivot and their client.
But within a week, the visit was arranged.
IGI surprised the little boy with a visit, creating a moment that was captured on film and had a profound emotional impact. As Liz recalled during our conversation:
“That is by far the best day in my professional career to this day. I got to connect with the family and learn that this tool has changed their lives. I got to learn that this stuffed animal was this little boy's best friend, because he lived in isolation all of his life. And it took on another level that I didn't know was possible. I didn't even know that what we were doing in this creative space of working within this community had such an impact.”
Today, more than a decade later, patients and advocacy organizations still request IGI and sales representatives continue to carry him. He is a practical tool that has stayed aligned with the needs of the people who helped shape him.
IGI proves that when you commit to co-creation and community-first solutions, marketers can create an enduring tool that generates long-term commercial value and helps improve the lives of patients.
As Liz puts it, "Great marketing, great tools should be enduring and stand the test of time." IGI sure has.
Ready to go deeper? Listen to the full episode with Eric Southwell, Paul Avery, and Liz Kanter on Spotify, Apple Podcasts, YouTube, or wherever you get your podcasts.
